All published articles of this journal are available on ScienceDirect.
KiOmedine® CM-Chitosan is Effective for Treating Advanced Symptomatic Knee Osteoarthritis up to Six Months Following a Single Intra-Articular Injection: A Post Hoc Analysis of Aproove Clinical Study
Abstract
Background:
Symptomatic knee osteoarthritis (OA) is typically treated with hyaluronan-based intra-articular injections. Advanced knee OA patients are often unresponsive to hyaluronan. KiOmedine® Carboxymethyl-Chitosan (CM-Chitosan), a novel fluid implant, was safe and effective for treating symptomatic knee OA.
Objective:
The objective of this study is to describe the efficacy of a single injection of KiOmedine® CM-Chitosan in advanced knee OA.
Methods:
Patients with advanced knee OA enrolled in the APROOVE trial and treated with KiOmedine® CM-Chitosan were identified: subgroup-1, BMI >30 kg/m2 and/or Kellgren Lawrence (KL) grade III (n=39), and subgroup-2, BMI >30 kg/m2 and KL-grade III (n=8). Within-group analyses were performed using the WOMAC scores and OMERACT-OARSI responder criteria at 3 and 6 months.
Results:
In both subgroups, significant improvements in all WOMAC scores were observed at 3 and 6 months (p<0.001 for all comparisons). A high responder rate was observed at 3 and 6 months in subgroup-1 (63.2% and 65.8%) and in subgroup-2 (57.1% and 62.5%).
Conclusion:
This post hoc analysis of the APROOVE trial showed that a single intra-articular injection with KiOmedine® CM-Chitosan could be an effective therapeutic option for patients with advanced knee OA.
Clinical trial registration number: Clinicaltrial.gov
identifier: Net30679208.
1. INTRODUCTION
Knee osteoarthritis (OA) is a chronic disease characterized by joint pain and stiffness, reduced joint motion, and muscle weakness [1-3]. Long-term consequences of knee OA involve reduced physical activity, deconditioning, and disability significantly affecting the quality of life. Globally, the prevalence of knee OA is 22.9% in individuals over 40, illustrating a large burden on the global health care system [4].
Recommendations for treating knee OA include a combination of pharmacological treatments (e.g., pain relief drugs) and non-pharmacological treatments such as viscosupplementation [5-9]. Patients with advanced knee OA present with one or more of the following phenotypes that are risk factors for viscosupplementation failure [10-12]: tri-compartmental OA, isolated/severe patella-femoral OA with pain (i.e. Patellar syndrome), BMI > 30 kg/m2, radiographic Kellgren and Lawrence (KL) grade III or IV. These advanced knee OA phenotypes largely align with the findings of the EUROpean VIScosupplementation Consensus Group (EUROVISCO) [11].
KiOmedine® CM-Chitosan is a novel biomaterial implant composed of 2.0% non-crosslinked chitosan derivatives of non-animal origin, obtained by proprietary chemistry after extraction from the edible white mushroom Agaricus bisporus. It is a single-injection fluid implant, suitable for intra-articular injection [13]. Compared to the cross-linked hyaluronan, KiOmedine® CM-Chitosan has a higher lubrication capacity and a higher ability to fight against oxidative stress [14]. The prospective, multicenter APROOVE study determined that KiOmedine® CM-Chitosan was a safe and effective option for the treatment of knee OA [15]. The objective of this post hoc analysis was to further describe the benefits of KiOmedine® CM-Chitosan in patients enrolled in the APROOVE study who were retrospectively identified with advanced knee OA.
2. MATERIALS AND METHODS
2.1. Overview of the APROOVE Trial
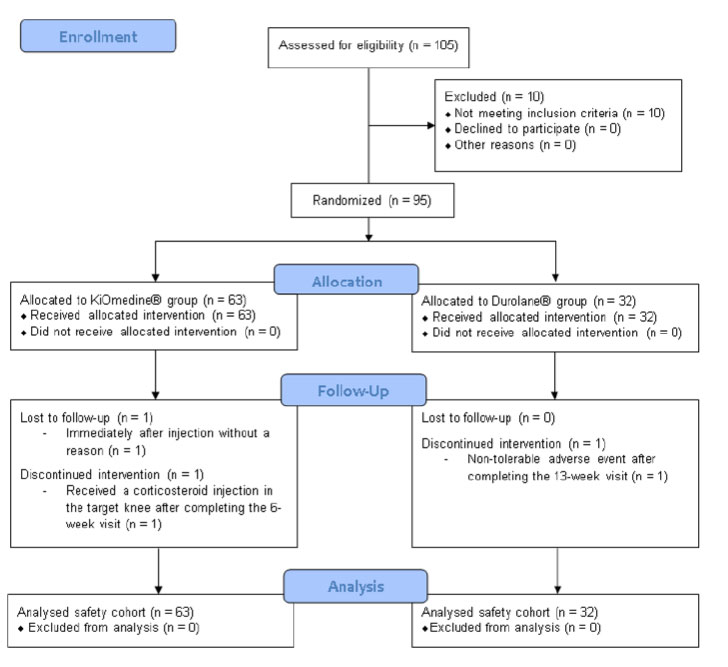
The APROOVE trial was conducted between October 2018 and January 2020 at four sites in the Netherlands and three sites in Hungary. The methodology has previously been described in detail (Fig. 1, ClinicalTrials.gov identifier: NCT03679208).
The trial included adult participants suffering from symptomatic knee OA and associated pain in the index knee for at least 6 months and who were either poor responders or unresponsive to simple oral analgesics (non-opioid analgesics and non-steroidal anti-inflammatory drugs). Participants were aged between 40-85 years, had a body mass index (BMI) ≤ 35 kg/m2, a KL grade II to III, and a baseline pain score of 7-17 on the 5-graded Likert WOMAC A after a mandatory 48-hour washout period of medication and minimal contralateral knee pain (i.e., WOMAC A ≤ 6). The participants received a single intra-articular injection of KiOmedine® CM-Chitosan (KiOmed Pharma) or Durolane® (Bioventus) and were followed for 6 months. The initial study trial was conducted in accordance with the ethical principles of Good Clinical Practice and the requirements of the Declaration of Helsinki. Favourable opinion/approval was obtained from the National Competent Authority and Central Ethics Committees in the targeted countries.
2.2. Subgroup Analysis
This post hoc analysis was focused on participants with advanced knee OA who were treated with KiOmedine® CM-Chitosan. The subgroup 1 included patients with a BMI >30 kg/m2 and/or KL-grade III. Within subgroup 1, we further described patients with a BMI >30 kg/m2 and KL-grade III (subgroup 2).
2.3. Endpoints
Clinical assessments included the self-administered Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index for pain, stiffness and function, the WOMAC total score [16-18]. The Osteoarthritis Research Society International (OARSI) Standing Committee for Clinical Trials Response Criteria Initiative and the Outcome Measures in Rheumatology (OMERACT) responder criteria for OA clinical trials were also used to evaluate response to treatment (an improvement in pain or in function ≥ 50% and an absolute change ≥ 20 were considered as a positive response to treatment) [19].
| Subgroups | Subgroup 1 | Subgroup 2: |
|---|---|---|
| Characteristics and treatment | BMI > 30 kg/m2 and/or KL grade III (n = 39) | BMI ≥ 30 kg/m2 and KL grade III (n = 8) |
| Age in years, mean (SD) | 63.3 (7.8) | 63.6 (7.8) |
| Female, n (%) | 33 (84.6) | 7 (87.5) |
| White/Caucasians, n (%) | 39 (100) | 7 (87.5) |
| BMI in kg/m2, mean (SD) | 30.2 (2.9) | 32.5 (1.8) |
| Disease duration in years, mean (SD) | 5.1 (6.4) | 3.7 (3.5) |
| Kellgren-Lawrence, n (%) | ||
| Grade II | 15 (38.5) | 0 (0) |
| Grade III | 24 (61.5) | 8 (100) |
2.4. Statistical Analyses
A within-group post hoc efficacy analysis was performed for subgroup 1 and subgroup 2. Missing values were neither replaced or extrapolated. All statistical analyses were performed using IBM SPSS Statistics (Version 21.0).
Descriptive statistics were used to characterize the KiOmedine® CM-Chitosan in different subgroups: number (n), mean and standard deviation (SD) for continuous variables and n and % for discrete variables. Performance differences for continuous variables between baseline and month 3 and between baseline and month 6 (WOMAC parameters) were evaluated in each of the subgroups using a paired Student’s t-test.
3. RESULTS
3.1. Demographics
A total of 63 patients were injected with KiOmedine® CM-Chitosan in the APROOVE study, of which 39 could be classified in the subgroup 1 (BMI >30 kg/m2 and/or KL-grade III) and eight in the subgroup 2 (BMI >30 kg/m2 and KL-grade III group). Data were missing for one patient at the 3-month follow-up visit in both subgroups. An overview of patient demographics and disease characteristics is provided in Table 1.
3.2. WOMAC
In subgroup 1, significant improvements were observed in the WOMAC total score and all subscales at 3 and 6 months (Table 2).
In subgroup 2, significant improvements in the WOMAC total score and all subscales were also observed at 3 and 6 months (Table 3).
3.3. Responders to Treatment According to OMERACT-OARSI
In subgroup 1, 63.2% (n=24) and 65.8% (n=25) of patients were responders at 3 and 6 months, respectively. The percentage of responders in subgroup 2 was 57.1% (n=4) and 62.5% (n=5) at 3 and 6 months, respectively.
| Variables |
Baseline N = 39 |
3 Months N = 38* |
3 Months p-value |
6 Months N = 39 |
6 Months p-value |
|---|---|---|---|---|---|
| WOMAC pain | 11.21 (2.52) | 4.55 (3.88) | <0.001 | 5.10 (4.43) | <0.001 |
| WOMAC stiffness | 4.18 (1.50) | 1.74 (1.79) | <0.001 | 2.00 (1.90) | <0.001 |
| WOMAC physical function | 36.97 (10.96) | 18.13 (13.56) | <0.001 | 18.46 (14.42) | <0.001 |
| WOMAC total score | 52.36 (14.32) | 24.42 (18.75) | <0.001 | 25.56 (20.31) | <0.001 |
| Variables |
Baseline N = 8 |
3 Months N = 7* |
3 Months p-value |
6 Months N = 8 |
6 Months p-value |
|---|---|---|---|---|---|
| WOMAC pain | 10.75 (2.12) | 4.57 (3.95) | 0.004 | 5.75 (5.49) | 0.011 |
| WOMAC stiffness | 3.88 (1.12) | 1.43 (1.98) | 0.009 | 1.25 (2.05) | 0.005 |
| WOMAC physical function | 33.00 (9.30) | 15.00 (12.49) | 0.007 | 17.13 (15.30) | 0.014 |
| WOMAC total score | 47.63 (11.40) | 21.00 (17.86) | 0.004 | 24.13 (22.49) | 0.009 |
4. DISCUSSION
In this post hoc analysis of the APROOVE data, we aimed to further describe the benefits of a single intra-articular injection of KiOmedine® CM-Chitosan in patients presenting with advanced symptomatic knee OA phenotypes. Pain, stiffness, and function improvements were observed at both the 3- and 6-month follow-up visits in patients with a BMI >30 kg/m2 and/or KL-grade III, as well as in patients with a BMI >30 kg/m2 and KL-grade III.
Patients with advanced OA phenotypes have been reported to respond less to viscosupplementation treatments [12]. For instance, a post hoc analysis of a randomized controlled trial assessing intra-articular injections of hyaluronic acid showed a diminishing rate of viscosupplementation success in obese compared to non-obese patients [20].
Conversely, patients with the advanced OA phenotypes included in this work responded well to KiOmedine® CM-Chitosan, and results were comparable to those already reported in the APROOVE study [15].
Limitations of our work are mainly related to the exploratory nature of the analysis and the small size of the subgroups. Moreover, because of APROOVE eligibility criteria, we could only include patients with BMI >30 kg/m2 and/or KL-grade III in this analysis. A clinical trial is ongoing to prospectively assess the impact of a single KiOmedine® CM-Chitosan intra-articular injection in a larger population of participants with advanced symptomatic knee OA (ClinicalTrials.gov identifier: NCT05214807).
CONCLUSION
In patients with advanced knee OA, KiOmedine® CM-Chitosan resulted in improvements in pain and patient-related function which were maintained for up to 6 months post-injection. KiOmedine CM-chitosan could be a good option to reduce the symptoms of patients suffering from advanced OA who are not eligible for TKA or who want to delay surgical intervention.
AUTHORS' CONTRIBUTIONS
Conceptualization, M.C., N.P. and M.S.; methodology, M.C., N.P., M.S. and Avania; validation, M.C., N.P. and M.S.; formal analysis, J.B. (independent statistician); investigation, P.J.E., G.S. and D.H.; resources, M.S. and M.C.; data curation, N.P., M.S. and Avania; writing—original draft preparation, Avania; writing—review and editing, M.C., N.P. and M.S.; visualization, Avania; supervision, M.S.; project administration, N.P and M.S.; funding acquisition, N/A. All authors have read and agreed to the published version of the manuscript.
LIST OF ABBREVIATIONS
| OA | = Knee Osteoarthritis |
| KL | = Kellgren and Lawrence |
| EUROVISCO | = EUROpean VIScosupplementation Consensus Group |
| WOMAC | = Western Ontario and McMaster Universities Osteoarthritis |
| OARSI | = Osteoarthritis Research Society International |
| SD | = Standard Deviation |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Slotervaart Hospital and Reade in the Netherlands (protocol code: NL66642.048.18; date of approval: September 05, 2018) and the Scientific and Research Ethics Committee of the Medical Research Council (MRC-SREC) in Hungary (protocol code: OGYÉI/43406/2018; date of approval: September 05, 2018). Due to unforeseeable circumstances at the Medical Center Slotervaart, unrelated to the study, the ethical dossier was transferred on November 06, 2018 to the medical ethical reviewing committee of the Isala Klinieken Zwolle, so as to serve as the central ethics committee in the Netherlands for the duration of the study. The transfer to the medical ethical reviewing committee of the Isala Klinieken Zwolle was approved on November 08, 2018.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Informed consent was obtained from all subjects involved in the study.
STANDARDS OF REPORTING
CONSORT guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data presented in this study are available on request from the corresponding author [N.P].
FUNDING
This research was funded by Research and Technological Innovation programs of Biowin, grant number 7360-Prouesse and the Walloon Region, grant number 7842-Kiogel and “The APC was funded by Kiomed Pharma”.
CONFLICT OF INTEREST
Dr. Gábor Skaliczki and Dr Daniel Haverkamp report no conflicts of interest that could impact the research. Dr Peter Emans and Dr Jacques Bentin are paid consultants for KiOmed Pharma . Mickaël Chausson, Nicolas Portelange, and Mathias Schifflers are full-time employees of KiOmed Pharma. Part of this article has previously been published in First-in-human Study to Evaluate a Single Injection of KiOmedine®CM-Chitosan for Treating Symptomatic Knee Osteoarthritis, in 2022, in The Open Rheumatology Journal, vol. 16, no. 1.
ACKNOWLEDGEMENTS
While the content of the manuscript was developed by the authors, the actual development of the manuscript was facilitated by Julia Fourie and Tjerk Zult from a third-party company called Avania.


